INTRODUCTION
- Full name: MEDIPHARCO PHARMACEUTICAL JOINT STOCK COMPANY
- Trade name: MEDIPHARCO
- Business license No. : 3300101406 (issued by Thua Thien Hue Planning and Investment Department on Jan 18 2006, 11th approval for modifying on May. 18 2020)
- Stock code: MTP_ Trading floor UpCOM – Hanoi Stock Exchange
- Charter Capital: 65.983.670.000 VNĐ
- Business fields:
- Manufacturing, trading and import-export medicines directly, medical equipment and supplies, cosmetic products, sanitary products, biological products, essential oils, aromatherapy.
- Preservation service of Medicines.
- Financial Investment; Market research and opinion polling services; Real estate business, land use rights of owners, users or lessees, Wine production and trading.
- Headquater:
- 08 Nguyen Truong to Street, Phuoc Vinh Ward, Hue City, Vietnam
- (+84) (0234). 3823099 – 3837731 - 3822701
- [email protected]
- medipharco.vn / medipharco.com.vn / medipharco.com

ORGANIZATIONAL STRUCTURE
HISTORY AND DEVELOPMENT
MILESTONES IN THE DEVELOPMENT PROCESS
THE NOBLE AWARD
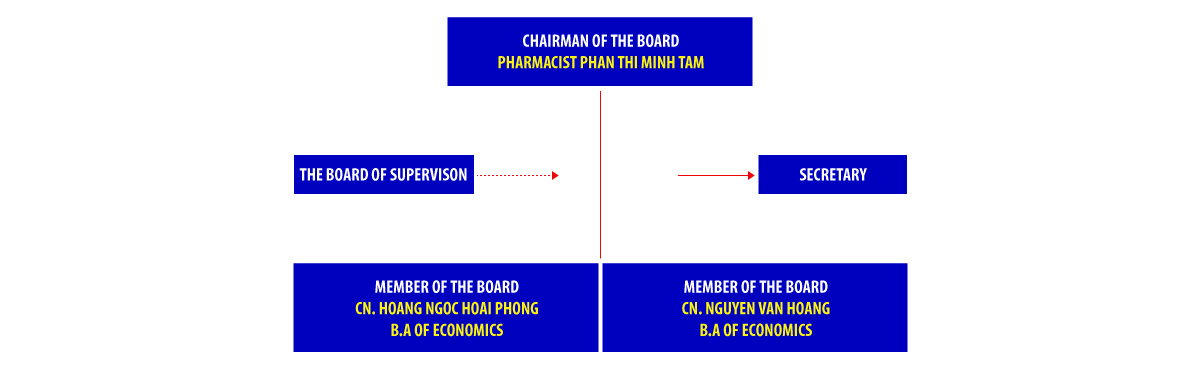
BOARD OF DIRECTORS TERM IV: 2020-2025
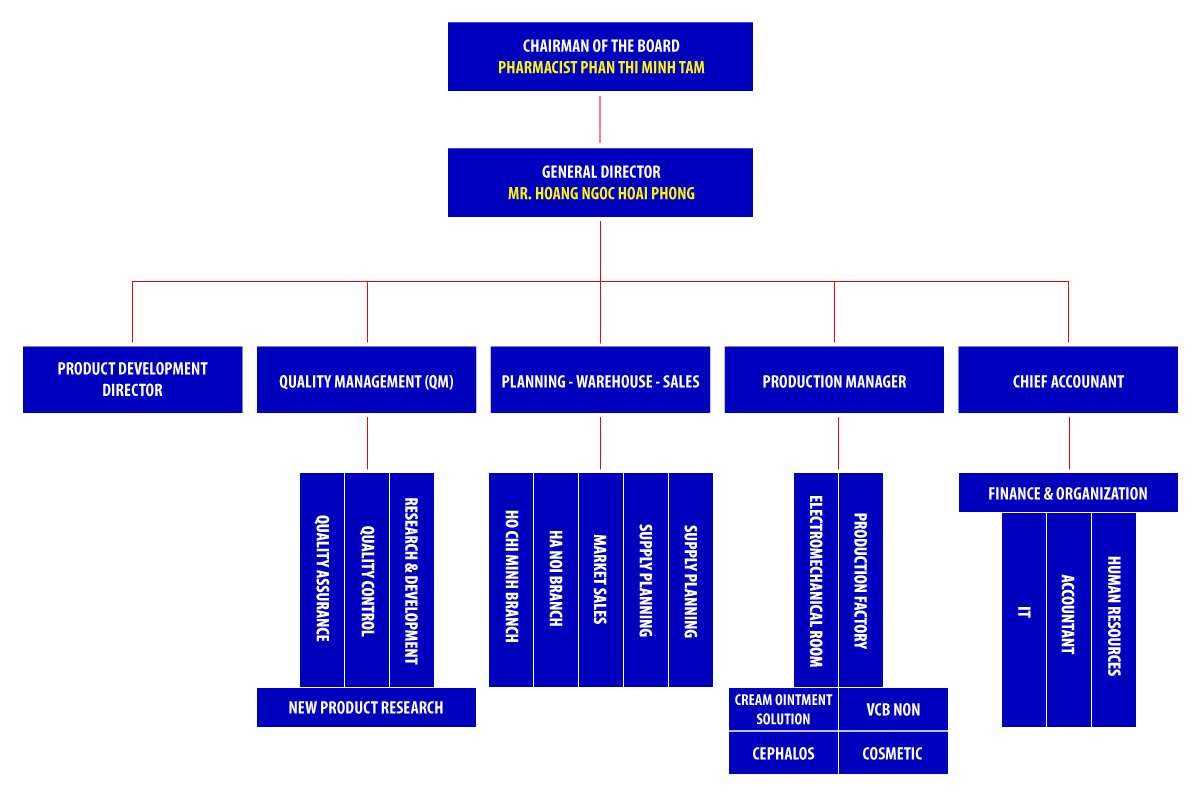
ORGANIZATION CHART
- Medipharco Pharmaceutical Company, formerly the Pharmaceutical Company of Thua Thien Hue province, was established April 8, 1976 after the day the South was completely liberated and unified, has gone through many periods with different names: Thua Thien Hue Pharmaceutical Company, Binh – Tri – Thien Pharmaceutical Union Enterprise, Thua Thien Hue Pharmaceutical Union Enterprise, Thua Thien Hue Pharmaceutical Company.
- In 1999, the Ministry of Health issued Decision No. 340/1999/QD-BYT dated September 2, 1999 accepting Thua Thien Hue Pharmaceutical Company as a member unit of Vietnam Pharmaceutical Corporation under the Ministry of Health and Renamed to Central Hue Pharmaceutical Company, its transaction name is MEDIPHARCO.
- Hue Central Pharmaceutical Company equitized its enterprise according to Decision No. 4751 / QD-BYT dated December 9, 2005 of the Ministry of Health changed its name to Medipharco Central Pharmaceutical Joint Stock Company.
- Approved by the State Securities Commission (according to Decision 175/UBCK-GCN dated September 20, 2007) on the issue of increasing charter capital, dated November 7, 2007 Department of Planning and Investment of Thua Thien province. Hue has granted the business registration number 3300101406 and changed its name to Medipharco -Tenamyd Central Pharmaceutical Joint Stock Company.
- Listing: on UPCOM – Hanoi Stock Exchange – MTP transaction code
- On July 12, 2011, the Chairman of Thua Thien Hue Provincial People’s Committee issued the Decision on granting Investment Certificate No. 311032000039 for the Investment Project to establish Medipharco Tenamyd BR S.r.l Pharmaceutical Joint Stock Company in Thua Thien Hue Province between the Company. Medipharco-Tenamyd Central Pharmaceutical Joint Stock Company & Tenamyd Cosmetics Joint Stock Company in Thua Thien Hue Province and BRUSCHETTINI Limited Liability Company – S.R.L / ITALIA. Medipharco-Tenamyd Central Pharmaceutical Joint Stock Company is the Parent Company of the Joint Venture in accordance with the Law on Enterprises.
- In 2017, the Company name changed to Medipharco Pharmaceutical Joint Stock Company
- From January 1, 2019, the General Meeting of Shareholders of Medipharco Pharmaceutical Joint Stock Company and the Joint Venture implemented the merger of Medipharco-Tenamyd BR s.r.l Pharmaceutical Joint Venture JSC into Medipharco Pharmaceutical JSC; Certificate of business registration by Thua Thien Hue Department of Planning and Investment of Thua Thien Hue Province No. 3300101406 – 10th change registration issued by Thua Thien Hue Department of Planning and Investment on January 3, 2019 after merger.
- 2003: Ministry of Health certified to meet GMP-ASEAN standard for Cream – Ointment – Solution production line
- 2006: Ministry of Health certified to meet GMP- WHO, GLP, GSP standard for production line:
- Ointment Gel (Eyes and Skin) Betalactam.
- Tablets, Granules, Powder, Betalactam-Free Oral Suspension
- Tablets, Granules, Cephalosporin Powder
- 2006: Meets ISO 9001: 2000 standard.
- From 2006 up to now:
- Continue to be certified by the Ministry of Health to meet the GMP-WHO, GLP, GSP standards.
- Thua Thien Hue Province Department of Health issuance of Certificate “Good Distribution Practices” standard (GDP, GPP) for Business systems at TT Hue.
- Received the certificate of “Good Distribution Practices” by the Department of Health of Hanoi and Ho Chi Minh City for the company’s branch
- 2020:
- The Department of Industry of Thua Thien Hue province issued the Certificate of Food Safety for the Wine production line of factory No. 1 – Phu Bai Industrial Park.
- Thua Thien Hue Department of Health issued a certificate of eligibility to produce cosmetics for factory No. 1 – Phu Bai Industrial Park
- Hand gel product registered at FDA-USA and certified with ISO 9001-2015
- 2005: The Nigerian Ministry of Health (NAFDAC) issued the GMP certificate for the topical cream production line.
- 2010: The Ministry of Health of Cuba issued the GMP certificate for Hebermin Burns
- 2006: Achieved Second Class Labor Medal
- 2011: Achieved First Class Labor Medal
- 2009: Emulation Flag by the Ministry of Health
- 2016: Emulation Flag by Thua Thien Hue Province
- 2016: Emulation Flag by Vietnam Health Union
- 2018: The Vietnam Health Union awarded the Emulation flag to the Unit with excellent comprehensive achievements in 2018